Abstract: Electrode materials have important influence on the working characteristics of solid oxide fuel cells (SOFCs). Double perovskite type A2BB'O6 oxide materials have very promising potential due to their strong oxygen ion transport capacity, low coefficient of expansion, good catalytic activity, strong resistance to sulfur poisoning and resistance to carbon deposition. The SOFC electrode material, with its double B-site element features, gives the material more structural and performance adjustability. This paper reviews the recent research progress of double perovskite-type oxide materials as SOFC electrode materials, focusing on the structural stability, electron and ionic conductivity, and electrocatalytic activity of double perovskite-type electrode materials, and points out that the current dual perovskite electrodes The main problems with materials, and put forward the main research direction in the future.
A solid oxide fuel cell (SOFC) is a device that directly converts chemical energy into electrical energy. It has advantages that cannot be matched by traditional power generation systems. It is highly efficient, clean, friendly to humans, and is the 21st century’s most Hope to replace a new energy device for thermal power generation. SOFC is an all-solid-state device whose electrolytes, anodes, cathodes, and connectors use different ceramic or cermet materials. The properties of these materials directly affect the output power characteristics and long-term operating stability of SOFCs. The improvement and improvement of the battery preparation process are the focus of research and development of high performance SOFC.
At present, NiO/YSZ composite ceramic is the most ideal anode material when using H2 as fuel. During operation of the fuel cell, the H2 atmosphere of the anode causes NiO to be reduced to Ni and dispersed on the surface of the YSZ particles. Ni is a good catalyst for destroying the H—H bond, making H2 into two H+, releasing two electrons, and H+ reacts with oxygen ions from the cathode to produce water. At present, under the condition that there is still a problem in the preparation and storage of hydrogen gas, the development trend of solid oxide fuel cells is to syngas (H2 and CO mixture) or hydrocarbon gas directly as fuel, and Ni/YSZ anode will catalyze The formation of C—C bonds results in carbon deposition, which leads to deterioration of cell performance, and because some of the impurities in natural gas, especially sulfur, react with Ni to form NiS, which causes the loss of catalysis due to sulfur poisoning; therefore, Ni/YSZ is not suitable. The oxidation reaction used to catalyze hydrocarbon gas is also not suitable as anode material of SOFC fueled with hydrocarbon gas.
People strive to find new anode materials that can directly catalyze hydrocarbon gases such as methane. When the commonly used perovskite-type cathode material La1-xSrxMnO3 is applied to an intermediate temperature-solid oxide fuel cell (IT-SOFC), the polarization resistance increases, which affects the output power of the battery. To solve this problem, many new cathode materials have been developed, such as Ln1–xSrxCoyFe1–yO3–δ (Ln=La, Pr, Nd, Sm, and Gd), but these materials have large coefficients of thermal expansion (≈20×10– 6/K), which is difficult to match with common electrolytes, and affects the practical application of new cathode materials. Therefore, the development of new cathode materials with low expansion coefficient has also become a research hotspot. Double perovskite structure materials have attracted people's attention and become new types. Candidates for cathode and anode materials.
1 Structure of double perovskite materials
The double perovskite structure is proposed for perovskite ABO3 type structure, and can be expressed as A2BB'O6, wherein A is an alkaline earth metal (Sr, Ca and Ba); B is a divalent or trivalent metal; B' is five The transition metal (usually Nb, Mo, W, and Te). Due to differences in the ionic radius and electronic configuration of the B-site, many times it is not an ideal structure (space group Oh6). Most cases will be distorted to form tetragonal crystals, orthorhombic crystals, or monoclinic crystals; at the same time, ion radius and ion Interactions have a great influence on the formation of different structures.
The structure of A2BB'O6 is similar to that of monolayer perovskite compound ABO3. There are similarities and differences between them: similarity is that they all have a stable skeleton structure, and the cations in the skeleton structure are substitutable and low-cost substitution. Oxygen vacancies or transition metal oxide valence states can produce defects, which can change the oxygen absorption and desorption properties and electrical conductivity, and improve the catalytic performance; the biggest difference is the eight atoms in the double perovskite structure. The surface structure is formed by alternate arrangement of BO6 and B'O6. Each B ion and B' ion are separated by oxygen ions to form a B-O-B' bond (see Figure 1).
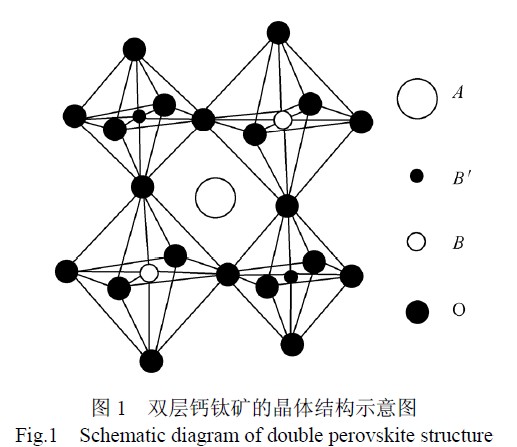
2 double perovskite anode materials
In the early studies, Sr2FeNbO6 exhibited relatively high electrical conductivity. The electrical conductivity at 900°C, 5% H2/Ar atmosphere can reach 2.39S/cm, and it has good chemical stability. In order to increase the oxygen vacancy concentration and increase the ionic conductivity, Xia et al. doped the divalent metal ions Cu2+ (r=0.087 nm) and Zn2+ (r=0.088) similar to the radius of Fe3+ (ion radius r=0.0785 nm) in the B position. (Nm), materials such as Sr2Fe1–xZnxNbO6–x/2 (0≤x≤0.5) and Sr2Fe1–xCuxNbO6–x/2 (0.01≤x≤0.05) were prepared. According to the principle of charge compensation, oxygen vacancies will be generated in the material. Increase the ionic conductivity of the material.
Thangadural et al. proposed a series of anion-defective perovskite-type oxides such as Ba2BB′O5.5 (B=Li, Na; B′=Mo, W, Te) doped with monovalent metals at the B site. O is mainly ion-bonded, while B'-O is predominantly covalent. The series of compounds mainly exhibit good ionic conductivity, and Mo-containing compounds show relatively good performance, probably because Mo has a d0 electron orbit and is easily deviated from the Mo-O octahedral center, thus reducing the oxygen ion mobility and making the ionic conductivity relatively high.
In recent years, Huang et al. demonstrated a B-ordered double perovskite material Sr2MgMoO6 (SMMO). The material has high oxygen ion conductivity and electronic conductivity, and has good resistance to sulfur poisoning and carbon deposition. And it has good catalytic activity for hydrocarbon fuels. Since SMMO is a mixed conductor, the electrochemical reaction takes place at the entire electrode/gas phase interface and is not limited to the three-phase interface, which will reduce the concentration polarization of the cell; however, the conductivity of this material depends on the reduction of the material. State, the conductivity of the sample prepared in 5% H2/Ar was very low without further reduction, but after reduction in 5% H2/Ar for 20h, the conductivity increased by 3 orders of magnitude, 800°C at 5°C. The conductivity in %H2/Ar can reach 4S/cm, and the conductivity in pure H2 is 10S/cm. The above phenomenon occurs because SMMO generates oxygen vacancies under low oxygen partial pressure, and each oxygen vacancy causes two Mo(VI) to be converted into Mo(V), so that the material shows good conductivity.
A single cell with La0.8Sr0.2Ga0.83Mg0.17O2.815 as the electrolyte, SrFe0.2Co0.8O3–δ as the cathode, and Sr2MgMoO6 as the anode is assembled. The power density at 800°C in dry H2 is 0.84. W/cm2 also has good power density in wet H2, H2/5% H2S, and power densities at 800C are 0.81 W/cm2 and 0.83 W/cm2, respectively.
In a wide oxygen partial pressure and temperature range, the SMMO phase structure and chemical properties are stable. The thermal expansion coefficient is 12.7×10–6/K (691K≤T≤1074K) and 11.7×10–6/K(382K≤T≤ 633K), Compatible with a wide range of electrolyte materials, without physical and chemical reactions. Bernuy-Lopez et al. reported that SMMO generates a large amount of oxygen vacancies in the reducing atmosphere above 900° C., resulting in partial decomposition into Ruddlesden-Popper phase, which increases the Mo:Mg molar ratio. After 12h reduction at 1200 °C, 5% H2/N2, Mo and MgO will appear, which will limit its application as a SOFC anode material.
The value of δ in A2BB′O6–δ, which is also the concentration of oxygen vacancies and the valence state of B′, plays a crucial role in the electrochemical performance of the material. Matsuda et al. tested Sr2Mg·MoO6–δ in Coulomb titration. The change of the valence of Mo and the concentration of oxygen vacancy when Sr is replaced by elements with different radii or valences. The results show that the valence and oxygen vacancy of Mo when doped with the same bivalent metals (Ba and Ca). The concentration did not change much. With the decrease of the A-site ion radius, the valence of Mo increased slightly, and the oxygen vacancy concentration decreased slightly.
In simple perovskites, donor doping with higher-valence ions at the A site can increase its conductivity. Ji et al. replaced a portion of Sr(SLMM) in SMMO with La, and doped and modified it to prepare a doped anode material. When the doped anode is not using hydrocarbon as a fuel, carbon deposition does not occur. Sulfur poisoning and other phenomena, and the performance of SLMM in wet CH4 is better than that of SMMO; however, the conductivity is slightly lower than that of SMMO, probably because the addition of La reduces the order of Mg/Mo. Marrero-Lopez et al. showed that La-doped SMMOs are prone to phase transitions, and above 700°C, SrMoO4 ​​and La2O3 heterophases are produced, which limits the practical application of this material.
The material preparation process also has a great influence on its performance. Marrero-Lopez et al. synthesized the SMMO by freeze-drying. The conductivity at 800° C. in 5% H2/Ar was 0.8S/cm, which was higher than that reported by Huang et al. (4S). /cm) To be small may be due to different synthesis methods. The crystal grains synthesized by the freeze-drying method must be fine, so that the grain boundary resistance of the material increases, resulting in a decrease in electrical conductivity; however, the fine crystal grains and large specific surface area are favorable for increasing the three-phase interface, thereby increasing the catalytic activity of the anode material.
Since Ni is a good catalyst for destroying the H—H bond, the introduction of Ni in the double perovskite material should increase its catalytic activity. Wei et al. studied the anode material of Sr2NiMoO6, the conductivity reached 67S/cm in H2, La0.9Sr0.1Ga0.8.Mg0.2O3–δ(LSGM) as the electrolyte, and Ba0.5Sr0.5Co0.8Fe0.2O3–δ. The cells assembled for the cathode showed good performance: the power density reached 819mW/cm2 in H2 at 850°C, and the thermal expansion coefficient was 12.14×10–6/K, which was very close to the LSGM electrolyte.
As Co has the function of improving electrical conductivity and catalytic activity, Huang et al. recently studied the performance of Sr2MMoO6 (M=Co, Ni) as SOFC anode material, and the oxygen vacancies produced by the materials in the anode atmosphere are limited. Electrolyte-supported single cell with La0.8Sr0.2Ga0.83·Mg0.17O2.815 as electrolyte, SrFe0.2Co0.8O3–δ as cathode, Sr2CoMoO6 as anode, and power in 800°C, H2 and humid CH4 The densities are 735 mW/cm2 and 527 mW/cm2, respectively, while Sr2NiMoO6 only has a higher power density in a dry CH4 atmosphere.
The reason for the higher power density of Sr2CoMoO6 compared to Sr2NiMoO6 in wet CH4 is due to the catalytic activity of Sr2CoMoO6 for fuel reforming. The reaction is as follows: ![]() However, in CH4, Sr2CoMoO6 generates a small amount of SrCO3 and SrMoO4 ​​impurities. The reason that the performance of Sr2NiMoO6 is worse than that of Sr2CoMoO6 is that the Ni-O octahedron is more stable than the Co-O octahedron, resulting in less oxygen vacancies for Sr2NiMoO6 and lower ionic conductivity, affecting the properties of the material.
However, in CH4, Sr2CoMoO6 generates a small amount of SrCO3 and SrMoO4 ​​impurities. The reason that the performance of Sr2NiMoO6 is worse than that of Sr2CoMoO6 is that the Ni-O octahedron is more stable than the Co-O octahedron, resulting in less oxygen vacancies for Sr2NiMoO6 and lower ionic conductivity, affecting the properties of the material.
In general, the development of double perovskite-type oxides as anode materials for SOFCs is still not mature enough, and the performance varies greatly. Different synthesis methods lead to a large gap in performance, among which materials containing Mo are relatively good. The performance; but due to the difference in the structure of the double perovskite structure and the single perovskite, in the study of double perovskite materials, the types, characteristics (such as ion size and price difference) and material structure of the doping elements, The relationship between the charge compensation mechanism and the conduction mechanism is not very clear, so it needs to be further understood and studied to obtain a dual perovskite anode with stable structure and excellent electrochemical performance.
3 Double perovskite cathode materials
The cathode material is different from the anode material in that it requires good stability and high electrical conductivity in a high-temperature oxidizing atmosphere; it also needs to have a porous structure, and it must also have good catalytic performance to reduce the polarization resistance; another cathode The material also has better chemical compatibility with the electrolyte material.
For the double perovskite cathode materials, there have been related reports previously. Due to the low conductivity of the double perovskite cathode materials, no attention has been given to people.
Tao et al. studied the structure, stability, and electrical conductivity of Sr2FeNbO6. The results show that the material has good structural stability over a wide range of oxygen partial pressure, and has a similar thermal expansion coefficient to many electrolyte materials; however, in air atmosphere Among them, the conductivity of the material is low, and the conductivity at 3.100 x 10–2 S/cm at 900°C. Because Co-O bonds are more covalent than Fe-O bonds, and Co-based cathodes have very high electrical conductivity and low-temperature oxygen reduction activity, Xia et al. studied the incorporation of Co in Sr2FeNbO6. The results show that: Co doping Into the increase of the conductivity of the material, Sr2Fe0.1Co0.9NbO6 at 750 °C polarization resistance of 0.74Ω · cm2, the highest current density of 88mA.
In the past, there have also been reported in the literature LnBaCo2·O5+δ(LnBCO) (Ln=rare-earth element) type 112 dual perovskite materials, but researches are mostly concentrated on the research of low temperature structure and magnetic properties. Recently, it has been found that the dual perovskite PrBaCo2O5+δ(PBCO) and GdBa·Co2O5+δ (GBCO) have unusual oxygen ion migration capabilities. At 300-500°C, the oxygen diffusion coefficient and surface exchange coefficient of PrBaCo2O5+δ are respectively When ≈10–5 cm/s and ≈10–3 cm/s are reached, and at 350°C, the oxygen diffusion coefficient and surface exchange coefficient of GdBaCo2O5+δ reach 3×10–7 cm/s and 2×10–6 cm, respectively. /s. Good surface exchange kinetics make these materials highly catalytically active.
Unlike the simple perovskite material, the A-site element of the type 112 dual perovskite material is highly ordered. Ln3+ and Ba2+ are ordered to occupy the A-site lattice and form alternating layers along the c-axis, and the oxygen vacancies are completely concentrated in the rare-earth ion layer. This type of double perovskite structure is shown in Figure 2 and it can be seen that: The ideal structure of the compounds is stacked along the c-axis in the order of [CoO2][BaO][CoO2][LnO[delta]]... and the oxygen vacancies in the [LnO[delta]] layer also show strong, Prescriptive trends.
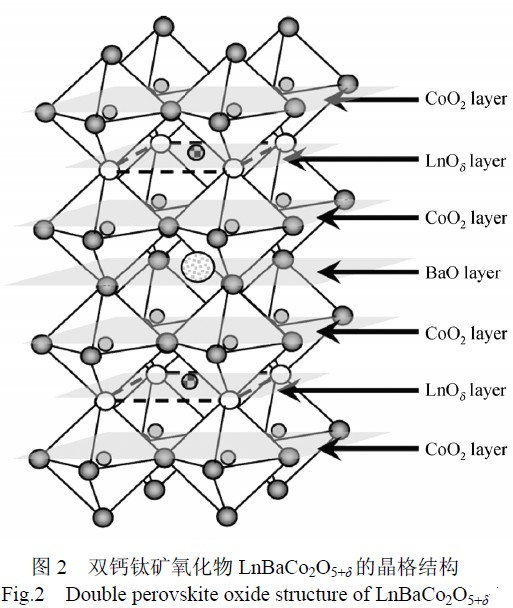
Compared with the simple perovskite with A-order disorder, the special distribution of oxygen vacancies in this layered perovskite provides a channel for the rapid migration of oxygen ions in the material, which can greatly promote oxygen ions in the material body. The diffusion may also provide more surface active sites for oxygen molecule reactions. Taskin et al. found that if a simple cubic perovskite with disordered A-sites is transformed into an ordered layered perovskite with A-site Ln3+ and Ba2+, then the diffusion of oxygen ions in the doped perovskite will be improved by several orders of magnitude. .
Co-based perovskite oxides have large thermal expansion coefficients due to the transition from low spin state to high spin state of Co3+, which results in lattice expansion due to the higher spin ionic radius than the lower spin ionic radius. . Some scholars believe that the formation of oxygen ion vacancies, resulting in the reduction of metal ions, ion radius increases so that the lattice expansion. Since GBCO has a large thermal expansion coefficient (20.1×10–6/K), it has hindered its development as a cathode material. Like simple perovskites, it is possible to consider doping some other elements (such as Fe, Ni, Cu, Mn, Cr, and Ti) at the Co site to reduce its thermal expansion coefficient. Jo et al. studied doping with Fe, Ni, and Cu at Co sites. The results show that the thermal expansion coefficient of GBCO after doping is significantly reduced (14.6×10–6/K).
Due to the unique structure of the dual perovskite structure materials, particularly the use of dual perovskite structure materials as cathode materials, there have been relatively few studies and some mechanism problems are not yet clear, so there is still much work to be done in depth.
4 Problems and Prospects
The development of double perovskite-type oxides as SOFC electrode materials is not mature enough, and the performance is very different. Although good catalytic activity is obtained, it is difficult to densify these materials in a reducing atmosphere. The study of conductivity and conductance of traces is less; at the same time, the understanding of the doping elements in the lattice structure, defect formation mechanism, and charge compensation mechanism of the material is not sufficiently deep, and the relevant experimental data is not comprehensive enough for the system. All these are It requires people to carry out more in-depth and detailed work, thus laying a theoretical foundation for the development of high-performance SOFC dual perovskite electrode materials.
Double perovskite-type oxides exhibit high catalytic activity for hydrocarbon fuels, and at the same time, they have low thermal expansion coefficient and good resistance to carbon deposition and sulfur poisoning; thus, they are a very promising type of SOFC. Electrode material. The current research focuses: on the one hand improving the conductivity of the material; on the other hand, the relationship between structure and performance is clearly defined, providing theoretical guidance for the selection of doping elements.
Although there are many problems mentioned above, as the research area continues to deepen, a batch of dual-perovskite electrode materials with good performance will continue to emerge. Since some double perovskite-type oxides exhibit lower conductivity, their electrical conductivity should be further increased on this basis. (Xie Zhixiang1, Zhao Hailei1,2, Zhou Xiong1, Shen Yongna1 1. Department of Inorganic Nonmetallic Materials, School of Materials Science and Engineering, University of Science and Technology Beijing, Beijing 100083, China; 2. Beijing Key Laboratory of New Energy Materials and Technology, Beijing 100083, China)
Acrylic Sign Board,Acrylic Sandwich Board,Acrylic Led Sign Board,Acrylic Light Board
NINGBO JIMING ELECTRIC APPLIANCE CO., LTD. , https://www.jimingemergencylight.com