Carbon atoms are magical, making up the world's softest mineral graphite and the hardest material diamond in nature. Recently, Chinese scientists have made breakthroughs in carbon atom research: a three-dimensional carbon structure, T-carbon, predicted by theoretical calculations by Professor Su Gang from the School of Physics of the Chinese Academy of Sciences, was successfully synthesized by a team of Chinese and foreign scientists. T-carbon, so that T-carbon becomes another three-dimensional new structure of carbon comparable to graphite and diamond.
On November 23, Su Gang said in an interview with the Science and Technology Daily that T-carbon is a fluffy carbon material with a large space available. If used as an energy storage material, its hydrogen storage capacity is not a weight percentage. Less than 7.7%. Su Gang believes that T-carbon will have broad application prospects in the fields of photocatalysis, adsorption, energy storage, and aerospace materials.
Since the 1980s, scientists have been interested in acquiring new structures of carbon and have given birth to two Nobel Prizes. This has not only had a huge impact in related fields such as chemistry, physics, materials and information science, but has also led to a large number of industrial and technological applications. Based on these structures, scientists have synthesized many new derivatives and made new functional devices and related products.
The carbon atom has four valence electrons, and after orbital hybridization (the original atomic orbits with relatively close energy are recombined into new atomic orbitals), it is like four hands, and has a strong combination with itself and other elements. ability. Carbon can form sp2 hybridized graphene, form sp3 hybridized diamond, and sp-sp2 hybridized graphite alkyne, sp-sp3 hybridized diamond alkyne. "Chemically, carbon can be combined with other elements, including DNA, proteins and other important biological macromolecules, making carbon one of the most basic elements of life on Earth," Su Gang said.
In 2011, Su Gang, the doctoral student Sheng Shenglei et al., after a lot of comparative studies, proposed that if each carbon atom in the cubic diamond is replaced by a tetrahedral structural unit composed of four carbon atoms, carbon will be formed. A new three-dimensional cubic crystal structure. Based on the first-principles study of density functionals, they found that the structure is extremely stable in terms of geometry, energy, and dynamics. They named the new allotrope of carbon as T-carbon. Studies have shown that T-carbon has the same space group as diamond, is a semiconductor with a direct band gap, can be controlled by doping to suit the photocatalysis. T-carbon also has a distinctive feature, the density is very small, about 2 / 3 of graphite, half of diamond.
Su Gang et al. found through calculation that T-carbon may form more easily under negative pressure conditions. T-carbon is likely to be observed in cosmic interstellar dust or extrasolar planets.
For the discovery of T-carbon work, industry experts highly praised that "T-carbon opens a new era of carbon structure research and will stimulate other scientists to conduct extensive theoretical and experimental research."
Can T-carbon be synthesized in the laboratory? Su Gang has been working hard to promote the experimental synthesis of T-carbon in recent years. At the beginning of 2017, the joint research team of Xi'an Jiaotong University and Nanyang Technological University in Singapore successfully irradiated multi-walled carbon nanotubes suspended in methanol solution by picosecond laser, and successfully realized the sp2 from the thermodynamic equilibrium state. The transition to the sp3 chemical bond, the formation of a new carbon material is completely consistent with the theoretical prediction of T-carbon, demonstrating the synthesis of T-carbon. The experimental results on the synthesis of T-carbon were published in Nature Newsletter not long ago.
Structure determines performance. This basic chemistry is the most magical manifestation of carbon materials. Changing the way carbon atoms are connected will result in very different properties. Graphite is a semi-metal and soft lubricant; diamond is non-conductive and is the hardest material; carbon nanotubes can be either semiconductor or metal, and at the same time the most strong fiber; similar to carbon nanotubes, graphene Several seemingly incompatible performances are among the most semiconductors with the highest carrier mobility. From synthetic diamonds to carbon nanotubes and graphene, carbon science has remained at the forefront of scientific research.
In 2011, scientists predicted the possibility of T-carbon by calculation, but it has never been observed and can be synthesized in the laboratory. Recently, Xi'an Jiaotong University School of Electric Power Electrical Insulation National Key Laboratory of New Energy Storage and Energy Conversion Nanomaterials Research Center Niu Chunming Qianren Team Zhang Jinying research team made a breakthrough in the research of carbon materials, synthesizing another new type of carbon A multi-walled carbon nanotube suspended in a methanol solution by a picosecond laser, successfully transforming from a sp2 to a sp3 chemical bond under the condition of extreme deviation from the thermodynamic equilibrium state, capturing this sub- Steady state structure.
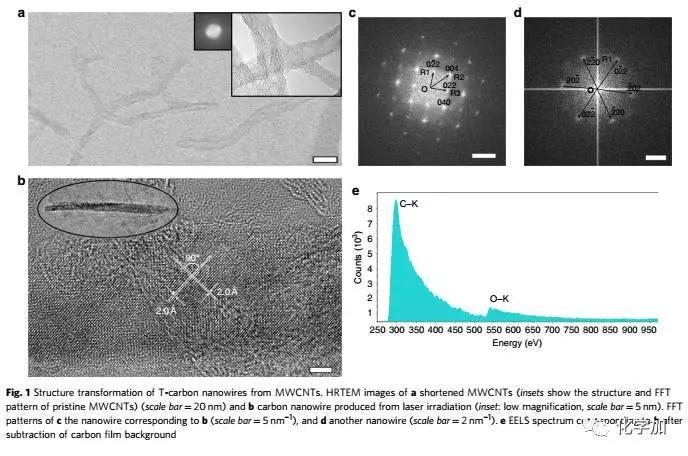
Detailed structural studies have found that hollow carbon nanotubes are converted into solid carbon nanorods under instantaneous femtosecond laser irradiation. The connection between carbon atoms in carbon nanorods is completely consistent with the theoretical prediction of T-carbon, which proves that this is synthesized. Kind of structure. The chemical reaction process of the experiment involves gas, liquid and solid three phases, and the reaction mechanism needs further study.
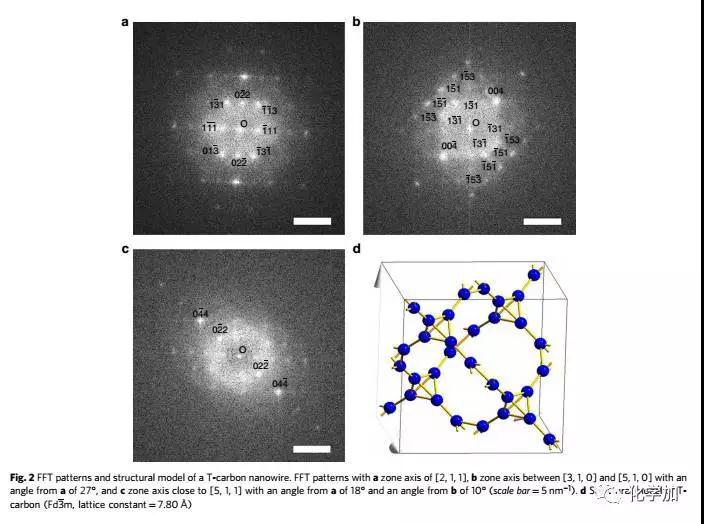
The laser experiment operation of the research was completed by the research team of Wang Wenjun of Xi'an Jiaotong University Mechanical College. The electron microscope work was completed by the team of Professor Jia Chunlin of Xi'an Jiaotong University's School of Telecommunications. The theoretical simulation work was completed by Su Haibin Research Team of Nanyang Technological University in Singapore. The related results are published in the journal Nature Communications (https://) under the title "Pseudo-topotactic conversion of carbon nanotubes to T-carbon nanowires in methanol under irradiation of picosecond laser". 9).
Stainless Steel Angle Seat Valve
Stainless Steel Angle Seat Valve,Sanitary Angle Seat Valve,Piston Angle Seat Valve,Steam Pneumatic Angle Seat Valve
ZHEJIANG KINKO FLUID EQUIPMENT CO.,LTD , https://www.kinkoflow.com